T Cell Immunology
T cells play a pivotal role in combating infectious diseases
The immune system is a complex network of cells and proteins that defend the body against infection. Key cells like T cells, B cells, macrophages, dendritic cells and neutrophils work together to identify, eliminate, and neutralize pathogens.
T cells, or T lymphocytes, are arguably the most important cells in the adaptive immune system, playing a central role in orchestrating almost all adaptive immune responses by:
- Eliminating infected or cancerous cells
- Mobilizing immune cells to mount robust immune responses
- Developing a pathogen memory to prevent reinfection
- Stimulating B cells to generate antibody responses
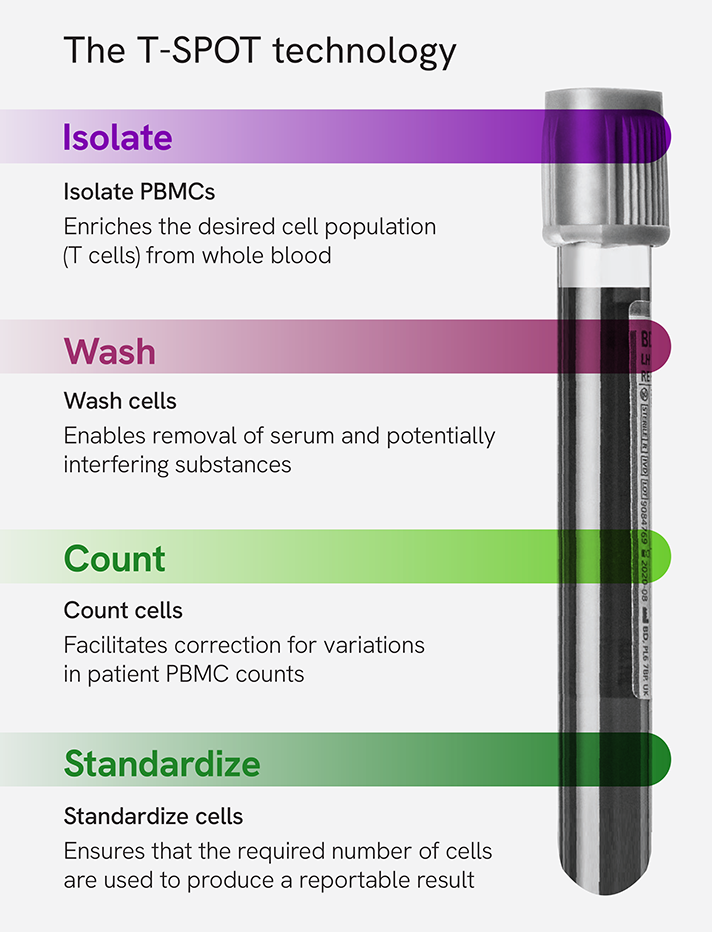
Our T-SPOT technology represents a unique approach to accurately measure T cell responses. By leveraging the ELISPOT methodology, it offers a refined analysis of individual immune responses to specific antigens. Unlike ELISA/chemiluminescent immunoassay (CLIA) methods, our technology includes:
- Cell isolation: It isolates peripheral blood mononuclear cells (PBMCs) from whole blood.
- Cell washing: It eliminates contaminants that could interfere with the cellular immune response and impact the assay's performance.
- Cell counting: It facilitates correction for variation in PBMC counts, ensuring the required number of cells are used to generate reliable results.
Through this innovative technology, we demonstrate our commitment to pushing the boundaries of healthcare and harnessing the power of the immune system to enhance diagnostic accuracy and improve patient outcomes.
Products may not be licensed in accordance with the laws in all countries. Please check with your local representative for availability. Revvity, Inc. does not endorse or make recommendations with respect to research, medication, or treatments. All information presented is for informational purposes only and is not intended as medical advice.
- National Institute of Allergy and Infectious Diseases. Overview of the Immune System.
- Abbas AK, et al. Cellular and Molecular Immunology. 9th edition. Elsevier; 2017.
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-v5. Abingdon, UK. November 2023.
- The World Health Organization. WHO operational handbook on tuberculosis: Module 3: Diagnosis - Tests for tuberculosis infection. Geneva. 2022.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Disease Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. 2017;64(2):111-115
- Wong SH, Gao Q, Tsoi KKF, et al. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis Thorax 2016;71:64–72.
- Egli A, et al. State-of-the-Art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55(12):1678-1689.
- Espigado I, Vicente F, et al. Timing of CMV-specific effector memory T cells predicts viral replication and survival after allogeneic hematopoietic stem cell transplantation. Transplant International. 2014;27(12):1253-1262.
- Abate D, Saldan A, Fiscon M, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010;202(4):585-594.
- Oxford Immunotec. T-SPOT.CMV Package Insert PI-CMV-IVD-UK-V3. Abingdon, UK. 2023.
- Oxford Immunotec. T-SPOT.COVID Package Insert T-SPOT.COVID-PI-UK-0001
- Oxford Immunotec. T-SPOT Discovery SARS-CoV-2 assay kit Package Insert PI-T-SPOT Discovery SARS-CoV-2–RUO-UK-V11
T cells play a vital role in diagnosing and monitoring infectious diseases.
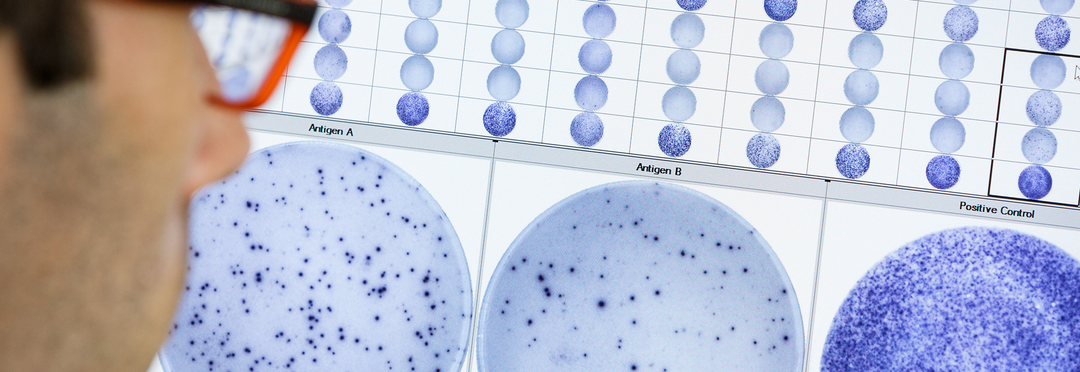
By utilizing an enzyme-linked immunospot (ELISPOT) assay, we can identify and analyze T cells unique responses to pathogens.
This allows for the precise identification of T cells that are responsive to specific antigens.
Tuberculosis
IGRAs are in vitro, blood-based assays that involve challenging the immune cells of the body with TB-specific antigens and then measuring the amount of interferon-gamma that is produced by the cells. The rationale is that any cell that recognizes the TB-specific antigens due to prior exposure, will produce interferon-gamma in vitro.
IGRAs are in vitro, blood-based assays that involve challenging the immune cells of the body with TB-specific antigens and then measuring the amount of interferon-gamma that is produced by the cells. The rationale is that any cell that recognizes the TB-specific antigens due to prior exposure, will produce interferon-gamma in vitro.
IGRAs have several advantages over skin tests; a single clinic visit is required, and they generally have high sensitivity and specificity. The WHO has now acknowledged that isolating, washing, counting and standardizing the cells used in IGRA testing can be beneficial, and these steps contribute to test reproducibility and performance.
The T-SPOT.TB test is a unique IGRA that requires only one visit and one tube. Unlike other IGRAs, the T-SPOT.TB test is standardized for both cell number and culture conditions, making it one of the most sensitive and specific tests for TB infection. By standardizing the number of cells and eliminating serum factors that could potentially impact test results, it ensures accuracy and reliability. Physicians can quickly and reliably diagnose TB infection in all patient groups, including the immunosuppressed.
Cytomegalovirus
Cytomegalovirus (CMV) is an opportunistic virus and common cause of morbidity and mortality in solid organ and hematopoietic stem cell transplant patients. T cell immunity against CMV is an important factor in maintaining viral latency and reducing susceptibility to disease.
Cytomegalovirus (CMV) is an opportunistic virus and common cause of morbidity and mortality in solid organ and hematopoietic stem cell transplant patients. T cell immunity against CMV is an important factor in maintaining viral latency and reducing susceptibility to disease.
Routine monitoring of an individual’s CMV specific T cell response may assist the evaluation of patients at risk of CMV disease and guide antiviral prophylaxis management.
The T-SPOT.CMV test uses the T-SPOT technology to assess a patient’s level of anti-CMV cell-mediated immunity by isolating and stimulating the immune cells with CMV specific antigens.
SARS-CoV-2
CE marked ror IVD use, the T-SPOT.COVID test is a standardized ELISPOT based technique for qualitative detection of a cell mediated (T cell) immune response to SARS-CoV-2 in human whole blood, intended for use as an aid in identifying and monitoring individuals with a T cell immune response to COVID-19 vaccination or SARS-CoV-2 infection.
CE marked ror IVD use, the T-SPOT.COVID test is a standardized ELISPOT based technique for qualitative detection of a cell mediated (T cell) immune response to SARS-CoV-2 in human whole blood, intended for use as an aid in identifying and monitoring individuals with a T cell immune response to COVID-19 vaccination or SARS-CoV-2 infection.
The test uses the established T-SPOT Technology with an optimized antigen mix, based on SARS-CoV-2 structural proteins, spike (S) and nucleocapsid (N), and allows the maximum breadth of the immune response to be measured.
For research-use only, the T-SPOT Discovery SARS-CoV-2 assay kit is intended to detect COVID-19 cellular mediated immunity using viral peptide pools from SARS-CoV-2. The assay kit is for research use only, and is not intended for diagnostic use in determining infection nor protective immunity towards any of the antigens tested.



























